Holographic
Imaging
|
MAS.450/854
Spring 2002
|
Holographic photochemistry: basic process (no bleaching)
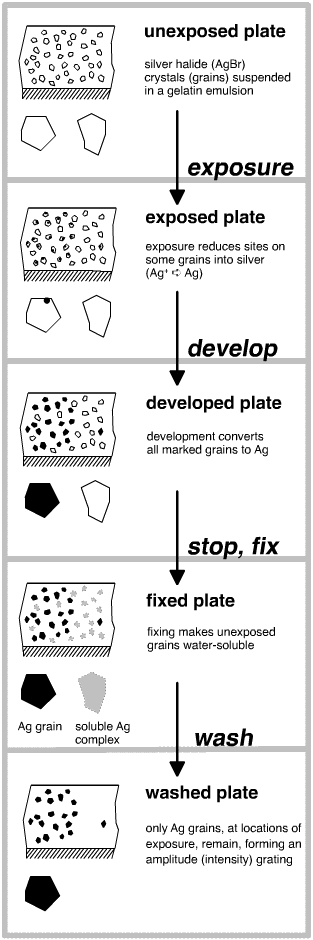 The
basics of holographic photochemistry are very similar to those of black and
white photography. Photosensitive plates are made of silver halide microcrystals
or "grains" (mostly silver bromide with some silver chloride) suspended
in a gelatin binder (together, they are called an "emulsion"), which
is in turn coated onto a glass or plastic substrate.
The
basics of holographic photochemistry are very similar to those of black and
white photography. Photosensitive plates are made of silver halide microcrystals
or "grains" (mostly silver bromide with some silver chloride) suspended
in a gelatin binder (together, they are called an "emulsion"), which
is in turn coated onto a glass or plastic substrate.
During exposure, some of the photons of light are absorbed by the silver halide
crystals and produce "electron-hole pairs," of which the photo-electron
is quite mobile, and the positively-charged silver ion is relatively stationary.
Some of the electrons eventually find their way to the surface and combine with
silver ions there to form tiny stable clusters of silver metal atoms, which
are the latent image "specks." As the exposure continues, photons
strike some part of more and more crystals.
Wet development amplifies the latent image by converting the silver halide
grains that have been marked with a silver speck entirely into metallic silver.
Silver grains of this small size appear black and opaque. Developers are weak
reducing agents - that is, they provide extra electrons during development.
Besides their reducing properties, developing solutions may also contain agents
that control their alkalinity, dissolve the silver grains slightly or interact
with the emulsion gelatin.
After the prescribed development time, the plate is placed in a dilute solution
of a weal acid (usually acetic acid), known as "stop bath." Most developers
are active only when alkaline, so the stop bath immediately halts the conversion
of silver-halide to metallic silver grains, and then rinses most of the chemicals
out of the gelatin.
After the stop bath, the plate moves to a solution of a silver halide solvent,
usually sodium hypothiosulfate or "hypo." This solution, called the
"fixer," dissolves the unexposed and undeveloped grains, leaving behind
only the black silver grains. At this point, the plate is no longer sensitive
to light. That is, the image is now fixed in place and won't change.
Following fixing, the plate is washed in filtered water. The water bath washes
out any soluble silver complexes and residual chemicals. Only metallic silver
grains remain in the emulsion. In black and white photography, this silver would
form the dark part of a photographic image. In holography, the silver grains
make up the fine-scale grating structure that will diffract light.
The final step is drying. When wet, the emulsion swells to five to seven times
its original thickness. As the water is removed, the gelatin shrinks dramatically
and "catastrophically." That is, the layer attempts to be either fully
swollen or fully shrunk, and water moves laterally through the gelatin to allow
this to happen. The result is that a drying "edge" or "front"
sweeps across the plate as it dries. Any dirt or flaw causes the front to "hang
up" and produce an isolated island of wet gelatin, which slowly dries and
leaves what is called a "water spot." In holograms, these water spots
become especially visible because of the distortion of the gelatin layer than
accompanies them. To minimize these effects, a small amount of surfactant (a
surface tension reducing agent) is added to a final rinse, so that the water
sheets off of the plate smoothly. Much care must be taken to avoid other drying
defects during this critical step!
 The
basics of holographic photochemistry are very similar to those of black and
white photography. Photosensitive plates are made of silver halide microcrystals
or "grains" (mostly silver bromide with some silver chloride) suspended
in a gelatin binder (together, they are called an "emulsion"), which
is in turn coated onto a glass or plastic substrate.
The
basics of holographic photochemistry are very similar to those of black and
white photography. Photosensitive plates are made of silver halide microcrystals
or "grains" (mostly silver bromide with some silver chloride) suspended
in a gelatin binder (together, they are called an "emulsion"), which
is in turn coated onto a glass or plastic substrate.